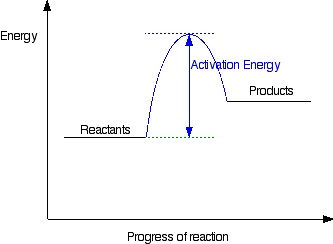
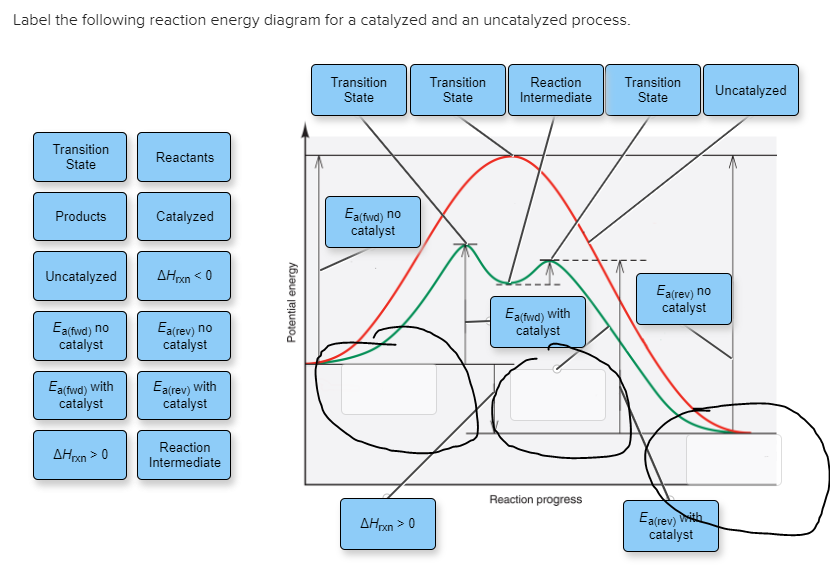
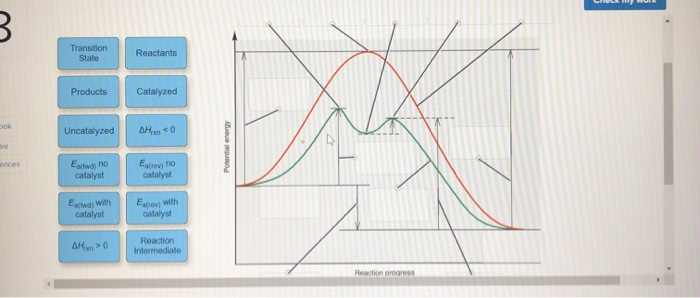
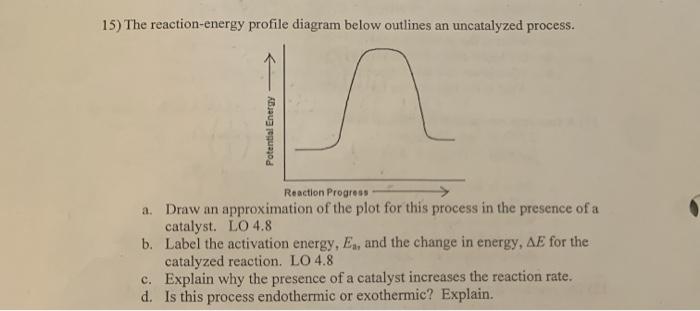
43 label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
SOLVED:Sketch a reaction energy diagram for an endothermic reaction ... SOLVED:Sketch a reaction energy diagram for an endothermic reaction that has both a catalyzed and an uncatalyzed pathway label the axes, the pathway for the catalyzed and uncatalyzed reaction. and indicate the positions of reactant product and transition state Mhm. The diagram will look something else. Chapter 8 BIOCHEM Flashcards | Quizlet Label the energy reaction graph for the following reaction showing the energy profile for a catalyzed and an uncatalyzed reaction. Ea, rxn not cat by enzyme, rxn cat by enzyme, energy released by rxn A.Look at the graph of reaction rate versus substrate concentration for an enzyme.In which region does the reaction rate remain constant? B.
Helicase - Wikipedia WebOne label is a fluorescent lanthanide chelate, which serves as the label that is monitored through an adequate 96/384 well plate reader. The other label is an organic quencher molecule. The basis of this assay is the "quenching" or repressing of the lanthanide chelate signal by the organic quencher molecule when the two are in close proximity – as they …
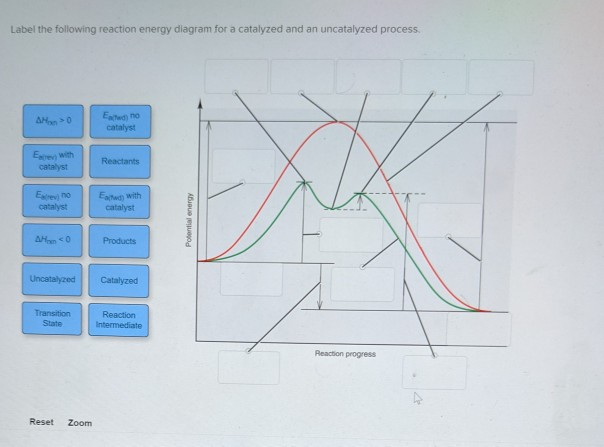
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
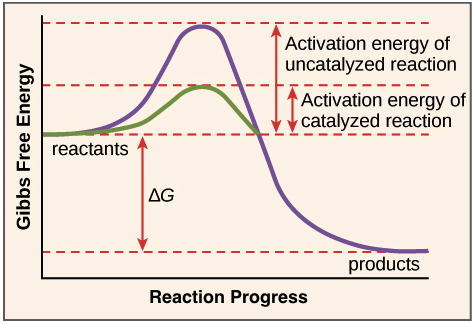
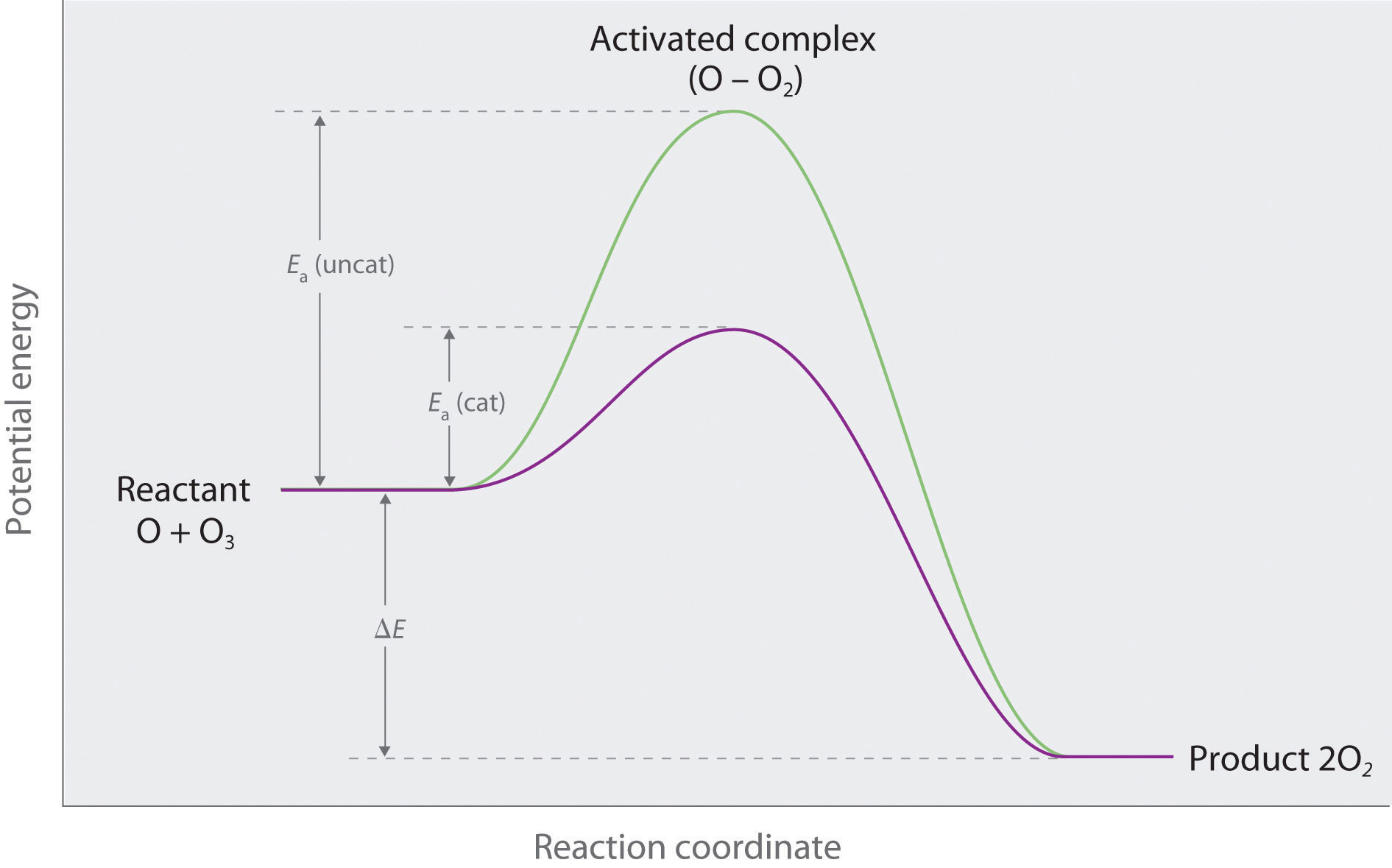
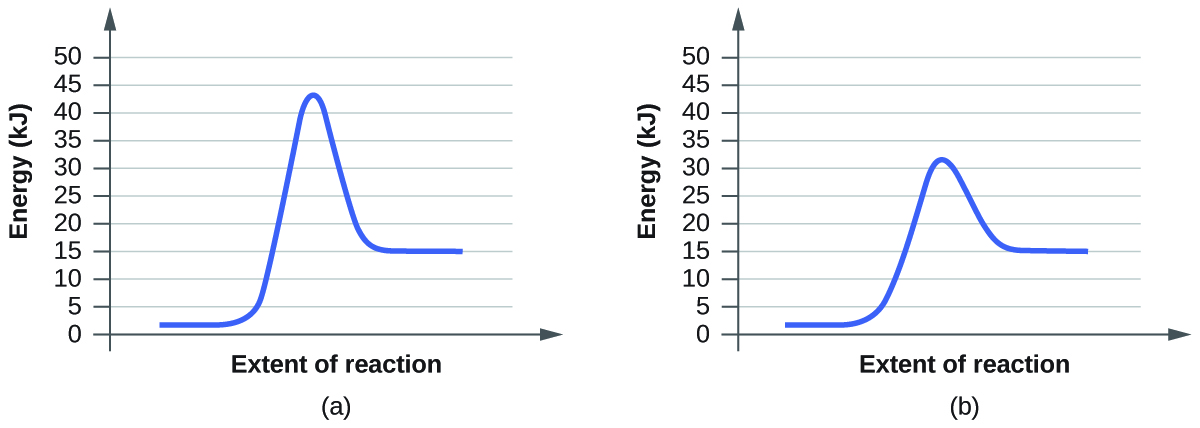
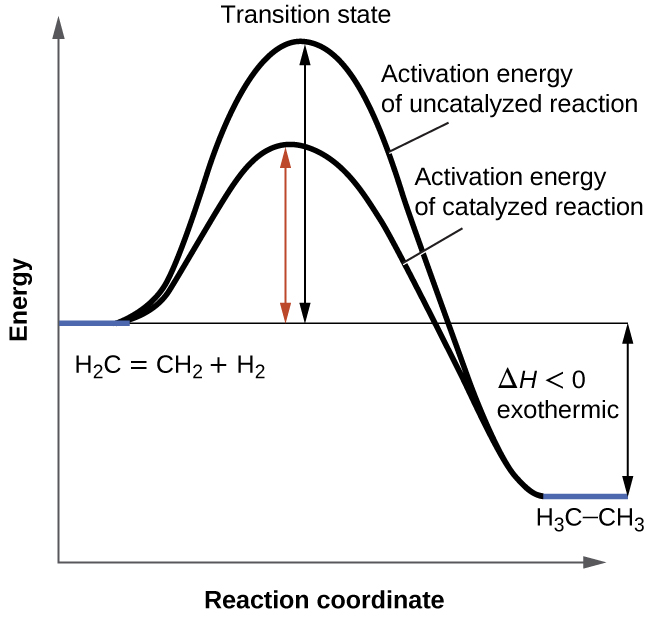
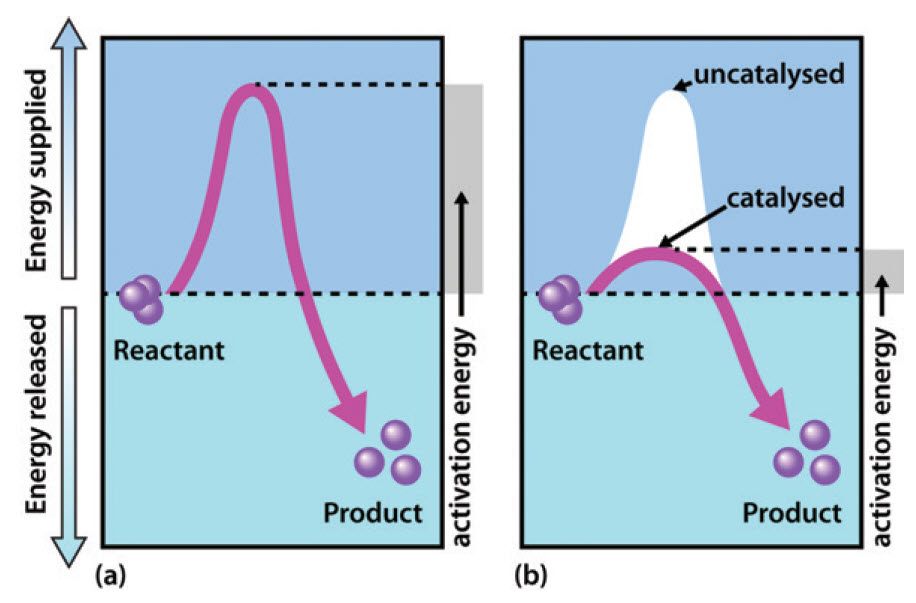
Catalysis | Chemistry for Majors - Lumen Learning Example 1: Using Reaction Diagrams to Compare Catalyzed Reactions. The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst. en.wikipedia.org › wiki › Cold_fusionCold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion. Biochemistry Mastering Ch. 8 Post-lecture Flashcards | Quizlet The enzyme urease catalyzes the hydrolysis of urea to ammonia plus carbon dioxide. At 28 ∘C the uncatalyzed reaction has an activation energy of about 123 kJ/mol, whereas in the presence of urease the activation energy is lowered to about 44 kJ/mol. By what factor does urease increase the velocity of the reaction?
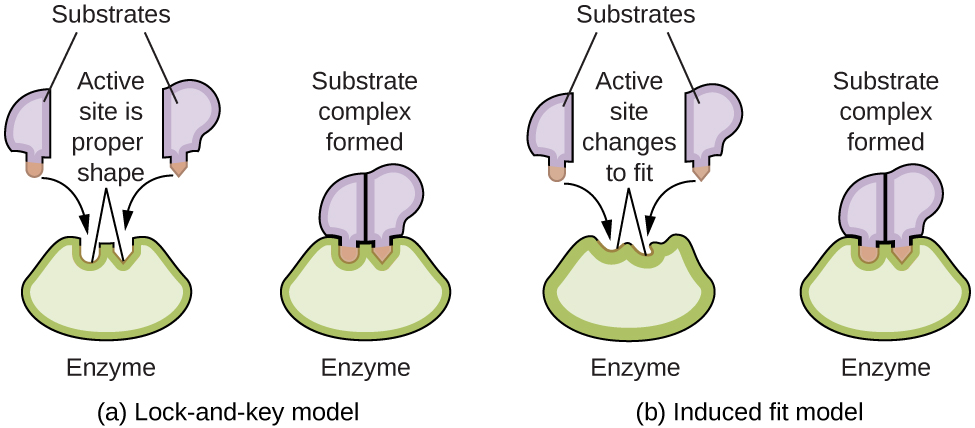
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.. BioChemistry Ch 8 Flashcards | Quizlet In an enzyme-catalyzed reaction, the reactant species to which the enzyme binds is called the substrate. The substrate is then converted into products by a series of steps. The lock-and-key model explains the steps involved in an enzyme-catalyzed reaction. Label the following diagram that illustrates the lock-and-key model of enzyme activity. Answered: Label the components of an energy… | bartleby Q: Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram.… A: The solution is provided in the next step: Q: Which of the following is NOT a reasonable assumption about the chemical reaction whose energy… A: Given answer of choices, The activation energy for the reaction is 100 kJ The reaction is… Q: B Comparing an uncatalyzed reaction to the same reaction with a catalyst ... A catalyst decreases the activation energy of a particular exothermic reaction by 57 kJ/mol, to 49 kJ/mol. Assuming that the mechanism has only one step, and that theproducts are 45 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions.What is the activation energy for the ... Chapter 8 Flashcards | Quizlet The enzyme urease catalyzes the hydrolysis of urea to ammonia plus carbon dioxide. At 21 C the uncatalyzed reaction has an activation energy of about 125 kJ/mol , whereas in the presence of urease the activation energy is lowered to about 46 kJ/mol . By what factor does urease increase the velocity of the reaction? 1.1 x 10^14
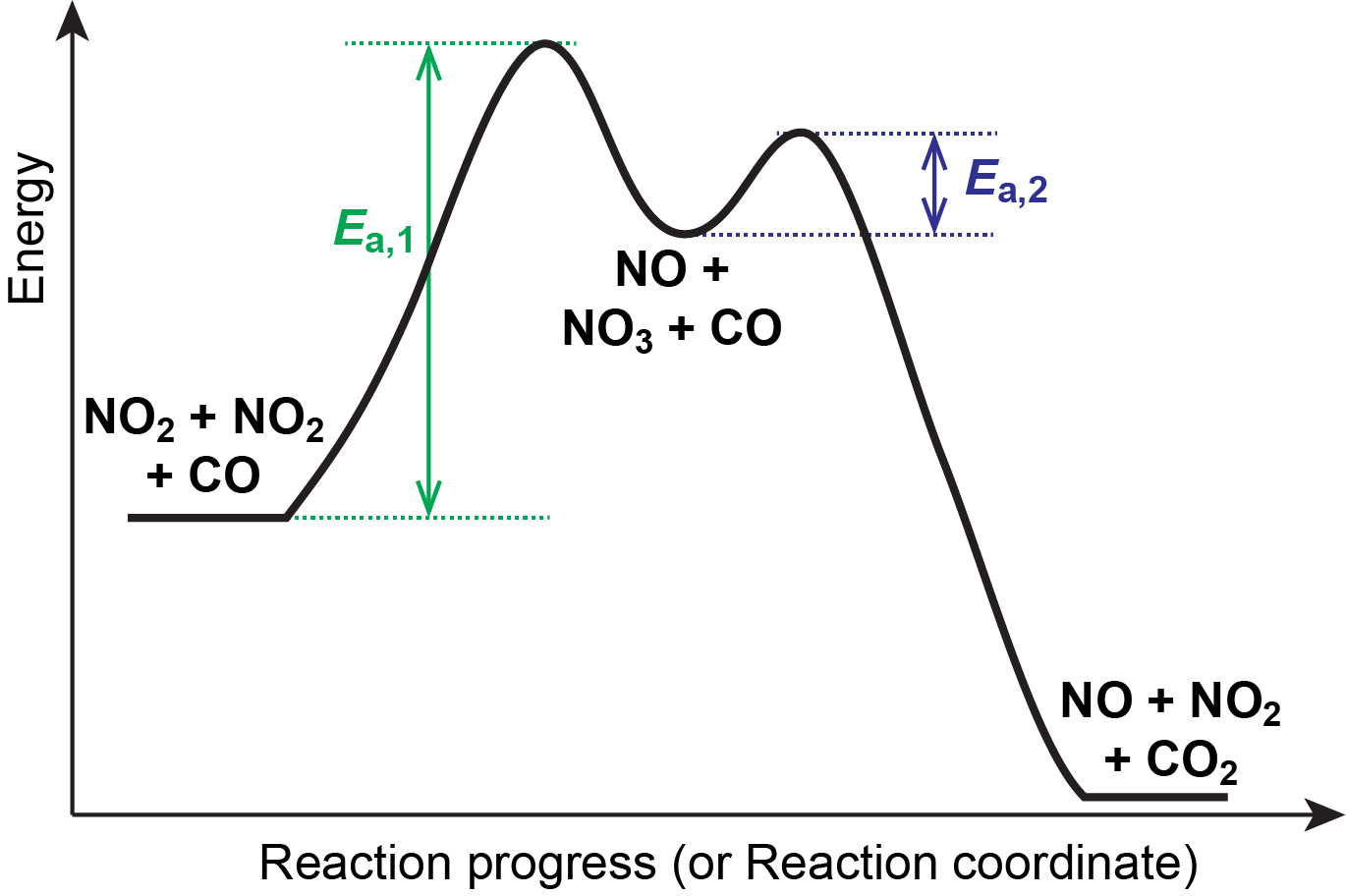
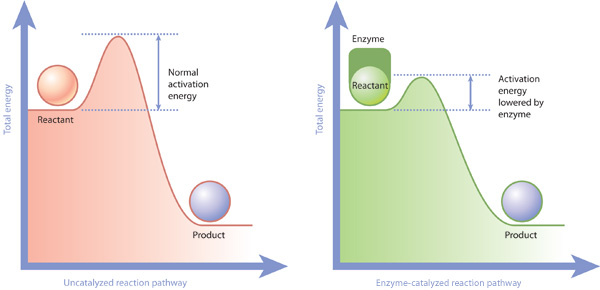
Mechanisms and Potential Energy Diagrams - Course Hero Figure 1. The potential energy diagram shows an activation energy peak for each of the elementary steps of the reaction. The valley between represents the intermediate for the reaction. From the CK-12 Foundation - Christopher Auyeung. The reaction whose potential energy diagram is shown in the figure is a two-step reaction. PDF AP CHEMISTRY 2006 SCORING GUIDELINES - College Board (d) Consider the four reaction-energy profile diagrams shown below. (i) Identify the two diagrams that could represent a catalyzed and an uncatalyzed reaction pathway for the same reaction. Indicate which of the two diagrams represents the catalyzed reaction pathway for the reaction. Diagram 1 represents a catalyzed pathway and diagram 2 Potential Energy Diagrams Answer-->. KNO 3 NH 4 Cl NH 4 NO 3 NaCl. 6/04. 21 A catalyst increases the rate of a chemical reaction by. (1) lowering the activation energy of the reaction (2) lowering the potential energy of the products. (3) raising the temperature of the reactants (4) raising the concentration of the reactants. Answer-->. PDF Energy/Reaction Coordinate Diagrams - chemconnections A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change ... Bonds are NOT in the process of breaking or forming. ! Energy Diagrams # Intermediates! 6! For each of the diagrams below, will the transition state ... Label each of those atoms nucleophilic (nu) or electrophilic (el) in each resonance ...
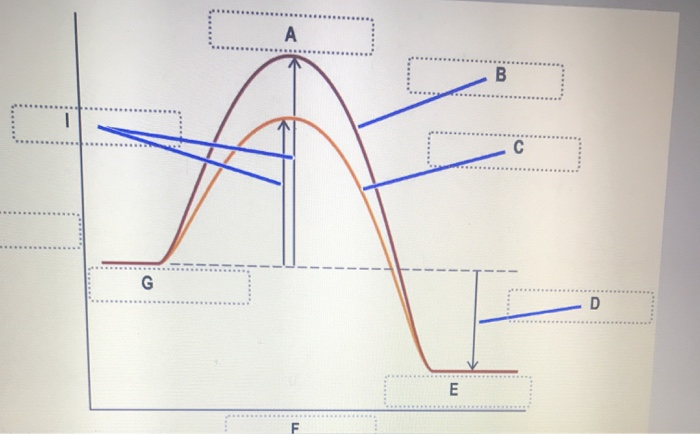
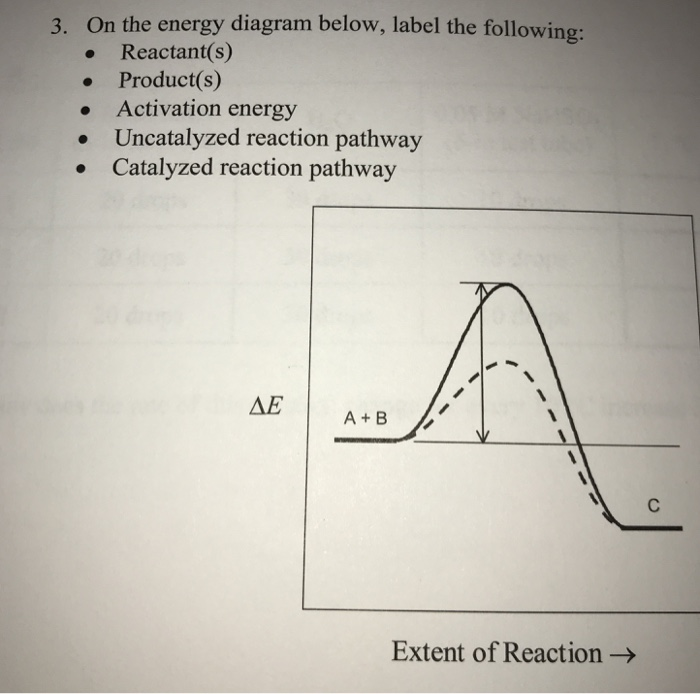
Answered: 2. A chemical reaction is initiated. As… | bartleby A chemical reaction is initiated. As time passes, the rate decreases. Explain. 3. On the energy diagram below, label the following: • Reactant(s) • Product(s) • Activation energy • Uncatalyzed reaction pathway • Catalyzed reaction pathway AE A+B Extent of Reaction → Answered: Sketch an energy level diagram for this… | bartleby Sketch an energy level diagram for this overall reaction, which is exothermic. Add to your diagram a second sketch for the uncatalyzed reaction. Clearly label which curve is catalyzed, and which is uncatalyzed. Also label the activation energy (EA) and the AH for each reaction. Cold fusion - Wikipedia WebCold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from … Biochemistry ; Questions and Answers - Academia.edu WebEnter the email address you signed up with and we'll email you a reset link.
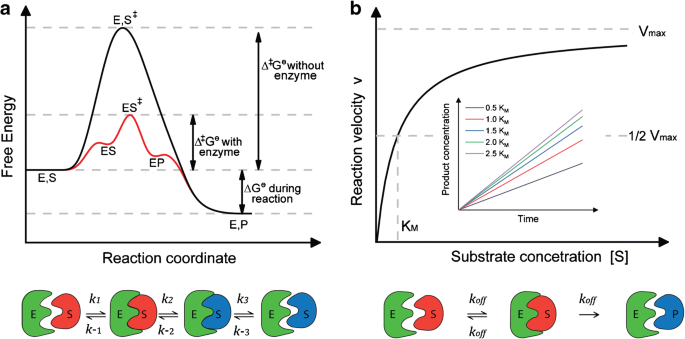
Energy diagrams for enzyme‐catalyzed reactions: Concepts and ... - IUBMB The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. The survey of several Biochemistry textbooks reveals a high diversity of profiles for the same process.
› 17849125 › Biochemistry_QuestionsBiochemistry ; Questions and Answers - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
The graph that best describes catalyzed and - Course Hero In the presence of a palladium catalyst, the ethyne forms ethene and ethane. This reaction is represented by the unbalanced equation C2H2(g) + H2(g) C2H4(g) + C2H6(g) + energy The energy diagram that represents both the catalyzed (---) and uncatalyzed reactions (___) is a. c. b. d. Upload your study docs or become a
› doc › 123745376Biochemistry PDF | PDF | Cell (Biology) | Biochemistry - Scribd The enzyme itself is not used up in the process, and is free to catalyze the same reaction with a new set of substrates. Using various modifiers, the activity of the enzyme can be regulated, enabling control of the biochemistry of the cell as a whole.
en.wikipedia.org › wiki › HelicaseHelicase - Wikipedia One label is a fluorescent lanthanide chelate, which serves as the label that is monitored through an adequate 96/384 well plate reader. The other label is an organic quencher molecule. The basis of this assay is the "quenching" or repressing of the lanthanide chelate signal by the organic quencher molecule when the two are in close proximity ...
SOLVED:Label the following reaction energy diagram for catalyzed and an ... VIDEO ANSWER:let's just ask this question. So here we need to label the following reaction energy diagram for the capitalized and unfertilized process. So let's draw this diagram. You know, we have this and here four C. This will be the levels open up McGee. So here we have an arrow, you will be in the middle, this will be the end over here and curie and So this would be the final 11 that we ...
tillery 8.25 Use energy diagrams to compare catalyzed and uncatalyzed ... Uncatalyzed Catalyzed Enzyme-substrate Complex In the above reaction, the lower curve is an enzyme-catalyzed reaction where the activation energy is notably lower than the uncatalyzed reaction. Suppose the enzyme in the diagram was mutated in such a way that its affinity for the substrate increased 100 fold, thereby affecting the enzyme ...
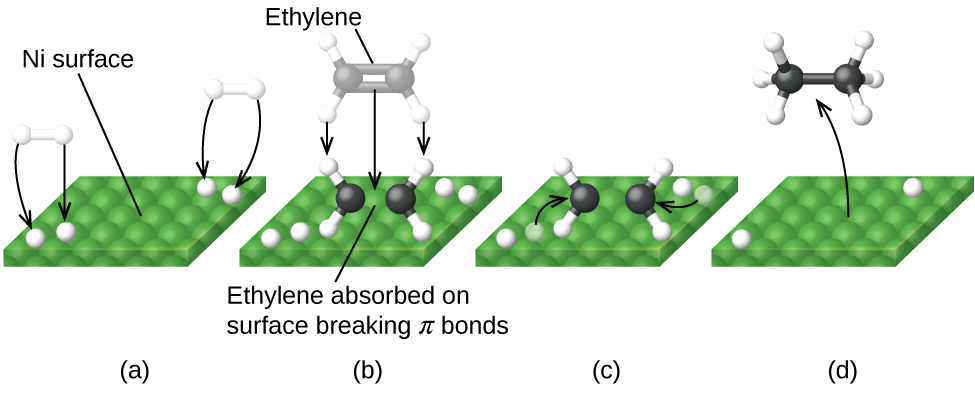
12.7 Catalysis | General College Chemistry II - Lumen Learning One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1.
Solved Label the following reaction energy diagram for a - Chegg Best Answer. 88% (42 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
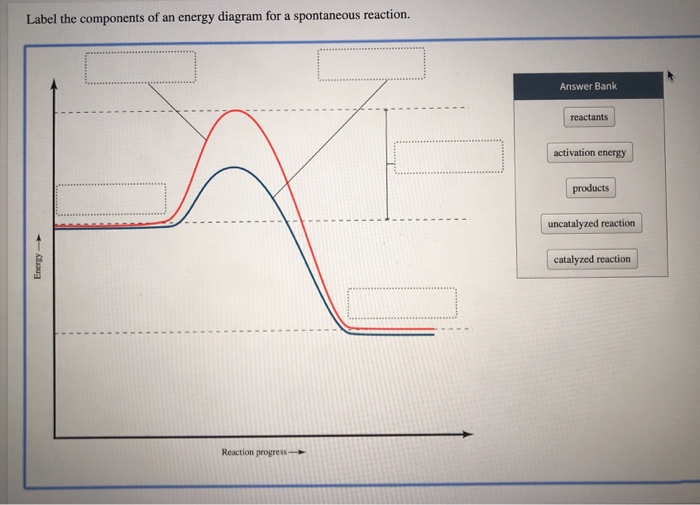
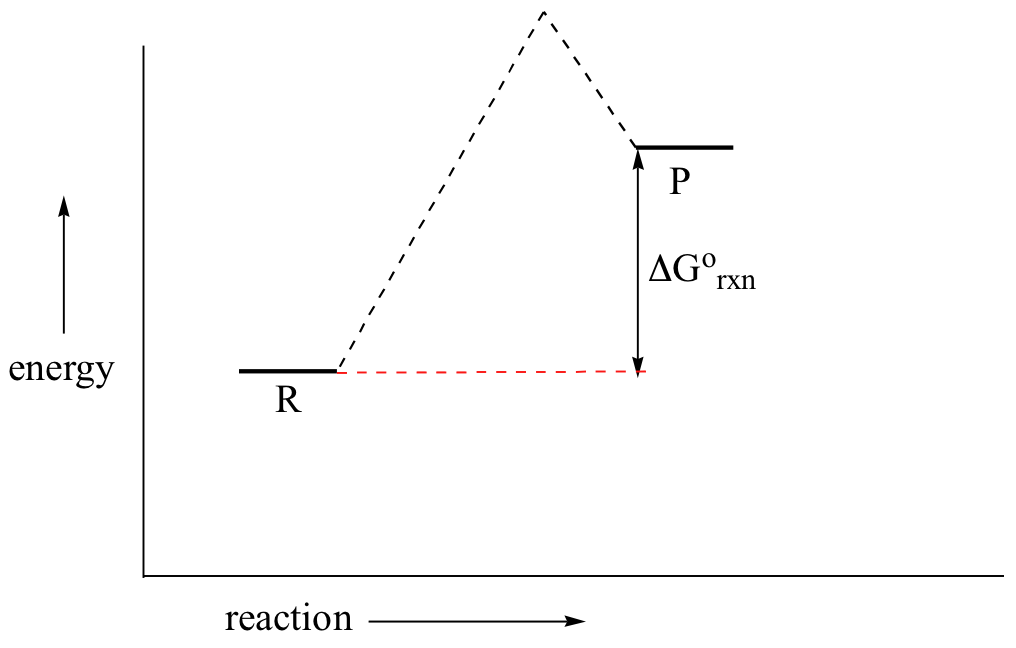
The diagram below represents a spontaneous reaction (deltaG degree < 0 ... Below is a figure depicting the energy diagram of a reaction. Both uncatalyzed and catalyzed version of the reaction are shown. Use the figure to answer the following questions. 5 1 AAG (the reduction in AG by the catalyst) G 2 3 A+B=P+Q Reaction coordinate a. Number 4 corresponds to which of the following? a. Products (P+) b.
Solved Label the following reaction energy diagram for a - Chegg Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. A > 0 Eatwa) no catalyst Exter, with catalyst Reactants Esinev) no Eastw with catalyst Potential energy Mono Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom This problem has been solved! See the answer
Answered: Sketch a qualitative reaction energy… | bartleby Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic. Note: Because the sketches are only qualitative, the energies in them don't have to be exact. They only have to have the right relationship to each other.
Biochemistry PDF | PDF | Cell (Biology) | Biochemistry - Scribd WebEduard Buchner contributed the first demonstration of a complex biochemical process outside of a cell in 1896: alcoholic fermentation in cell extracts of yeast. Although the term biochemistry seems to have been first used in 1882, it is generally accepted that the formal coinage of biochemistry occurred in 1903 by Carl Neuberg, a German chemist. Previous …
Solved Label the following reaction energy diagram for a | Chegg.com Expert Answer 100% (66 ratings) All the labeling you have done are correct except Hrxn < 0. Hrxn<0 because i … View the full answer Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
Labeling an Energy Diagram Diagram | Quizlet Labeling an Energy Diagram. STUDY. Learn. Flashcards. Write. Spell. Test. PLAY. Match. Gravity. Created by. Corey_Williamson PLUS. Terms in this set (9) Reactants. Starting ingredients for Forward reaction. ... Potential Energy of Products for FORWARD reaction and Potential Energy of Reactants for REVERSE reaction.
Answered: Label the components of an energy… | bartleby A: Alcohols react with acetic anhydride to form an ester. In a sugar the hydroxy group reacts with…. Q: 1. Write the balanced equation of the reaction between Al and oxygen in the presence of heat. 2.…. Balanced chemical equation occurs when the number of the different atoms of elements in the…. Question 25 KSCN is commonly used as the ...
Biochemistry Mastering Ch. 8 Post-lecture Flashcards | Quizlet The enzyme urease catalyzes the hydrolysis of urea to ammonia plus carbon dioxide. At 28 ∘C the uncatalyzed reaction has an activation energy of about 123 kJ/mol, whereas in the presence of urease the activation energy is lowered to about 44 kJ/mol. By what factor does urease increase the velocity of the reaction?
en.wikipedia.org › wiki › Cold_fusionCold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion.
Catalysis | Chemistry for Majors - Lumen Learning Example 1: Using Reaction Diagrams to Compare Catalyzed Reactions. The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst.


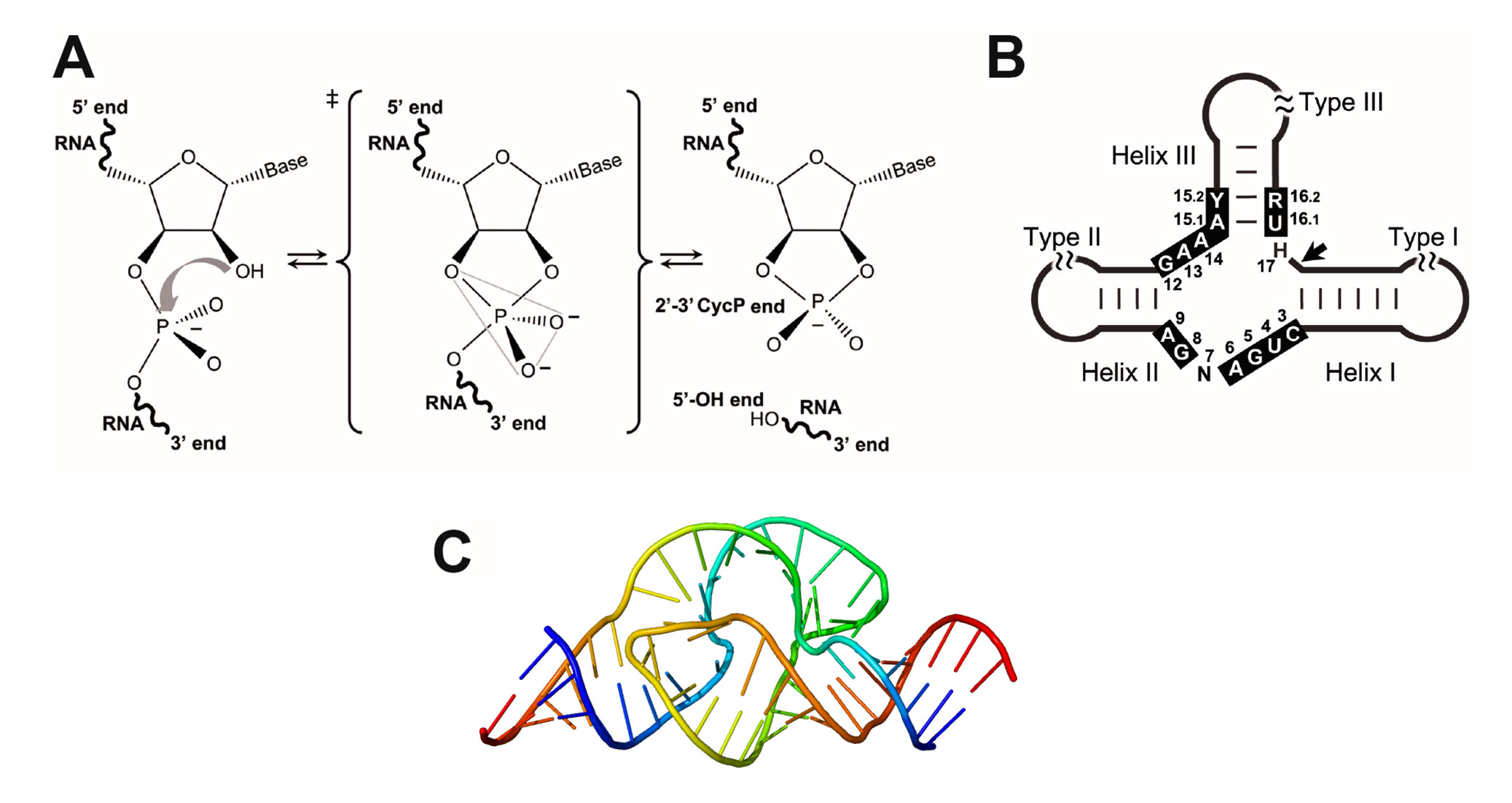
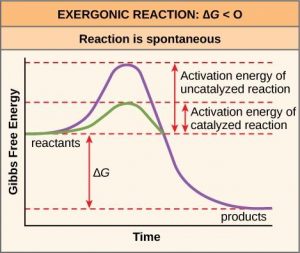

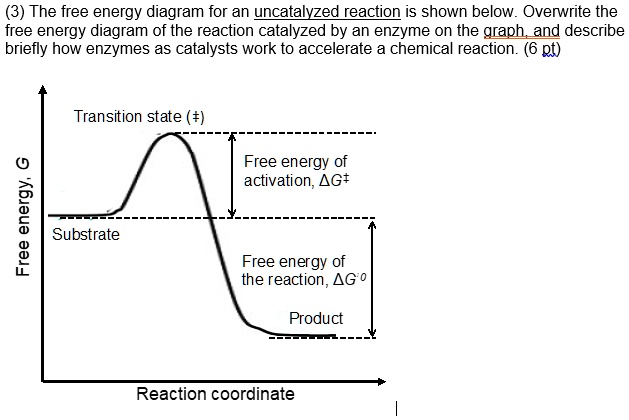



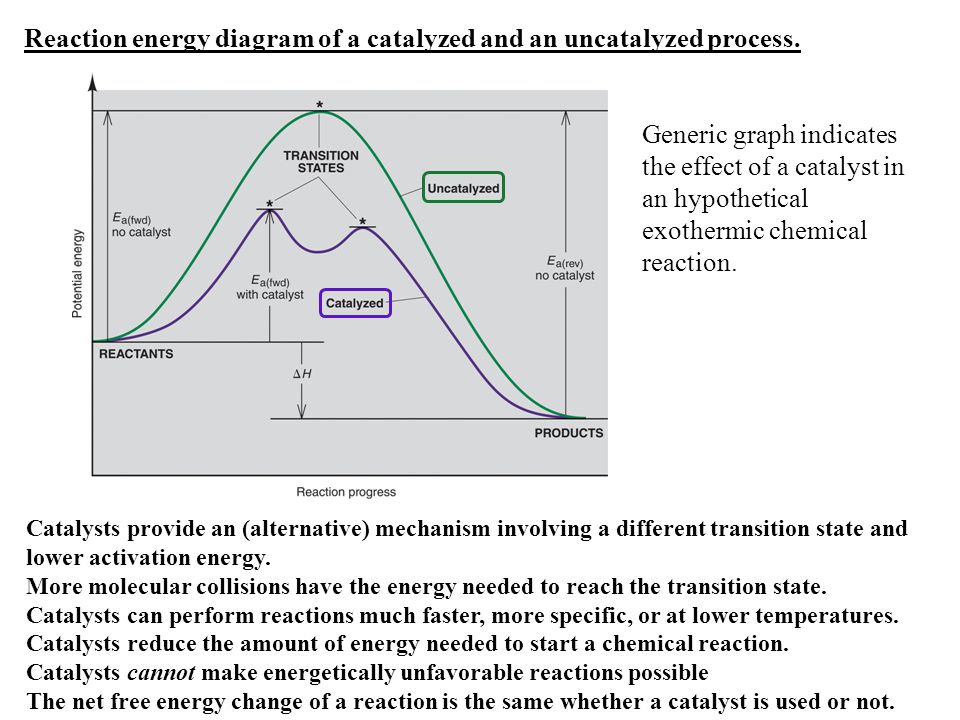









Post a Comment for "43 label the following reaction energy diagram for a catalyzed and an uncatalyzed process."