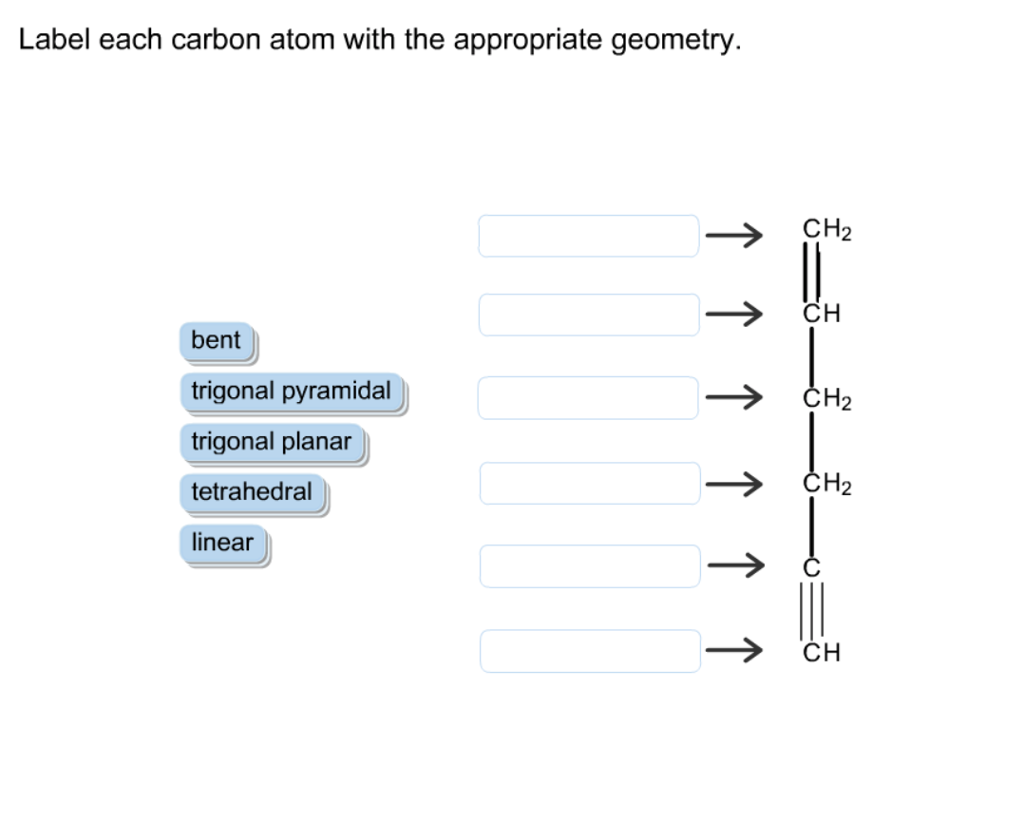
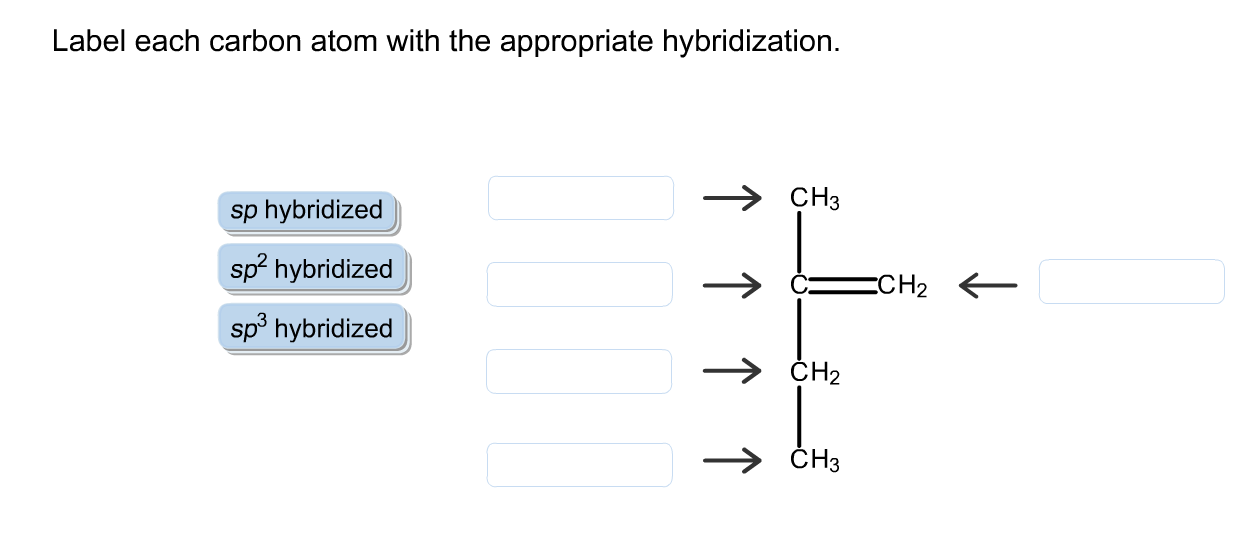
41 label each carbon atom with the appropriate geometry
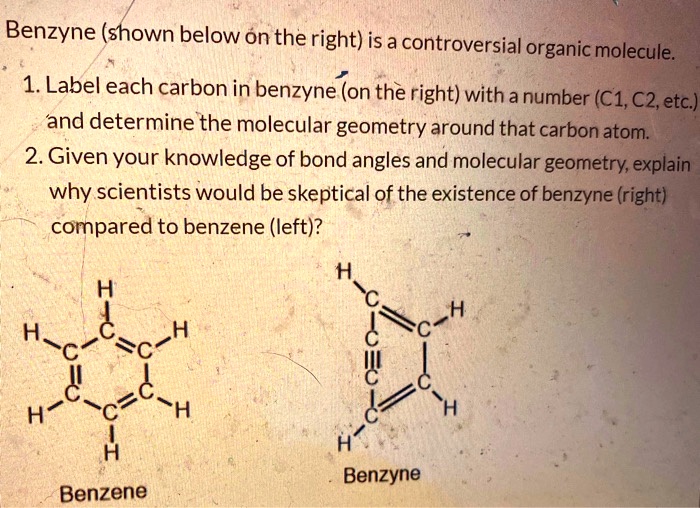
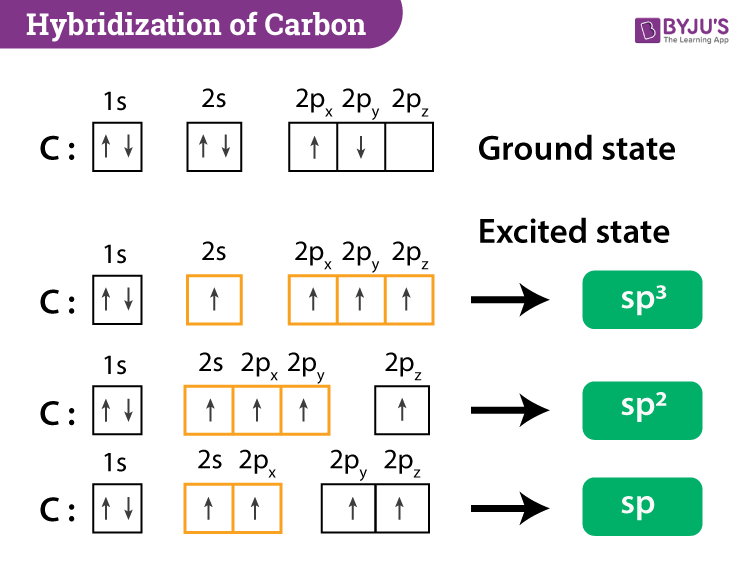
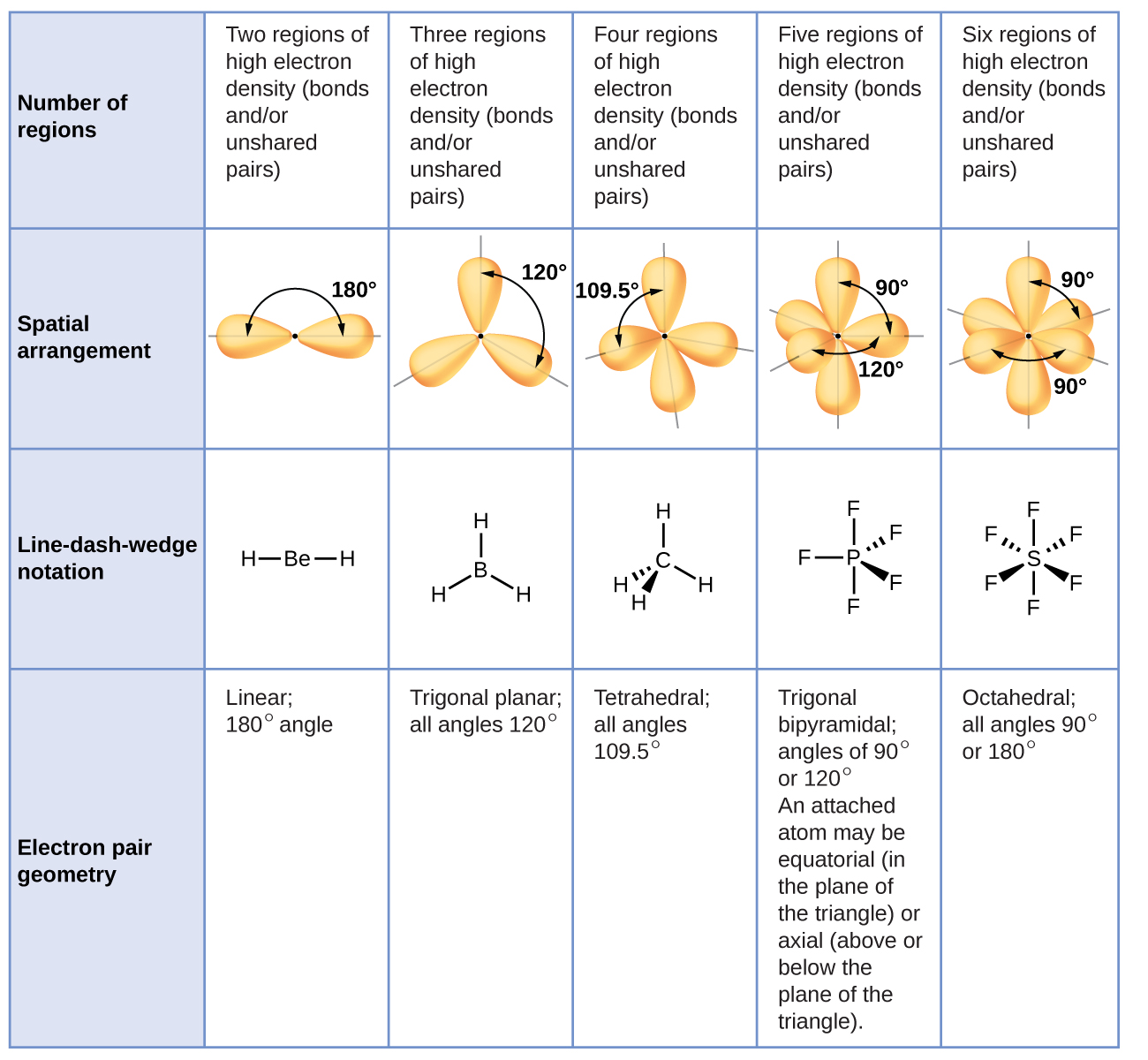
Drag Each Label to the Location of Each Structure Described Place each label on the appropriate cerebral lobe. Drag and drop the text labels onto the boxes next to the heart diagram. Correctly label the following major systemic arteries. Each label can be used more than once. Drag and drop each label identifying the cerebral area that if injured would result in the functional disturbance described. Label each carbon atom with the appropriate geometry ... - Course Hero The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. Fundamentals The geometry of sp3 hybridized carbon atom is tetrahedral. The geometry of sp2 the hybridized carbon atom is trigonal planar. The geometry of sp hybridized carbon atom is linear.
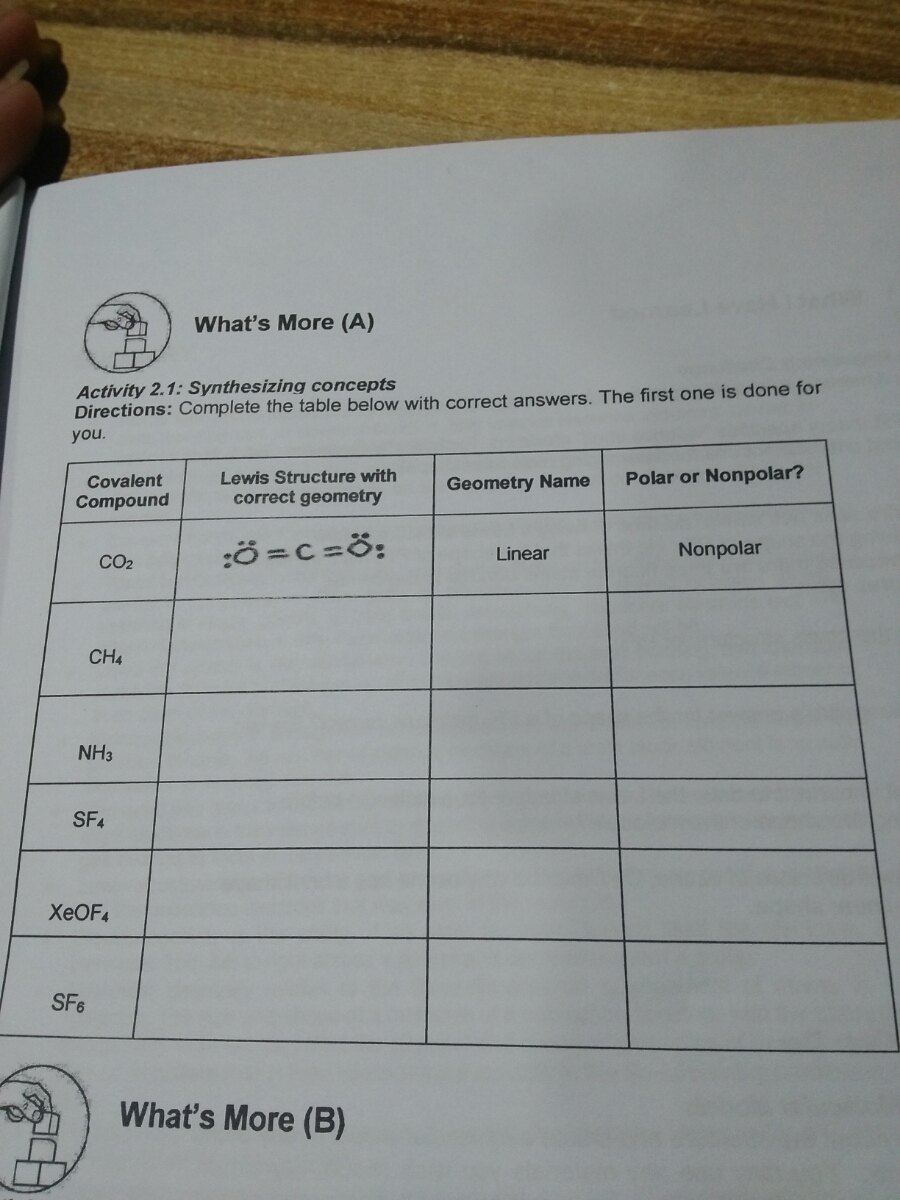
OChem Spring 2017 Exam 1 Flashcards - Quizlet Label each carbon atom wiht the appropriate geometry (cover right side) Sapling Hw Ch 1.21. Predict the molecular shape of methane, the carbonate ion, carbon dioxide, and the sulfite ion (cover left side of screen) ... Determine the hybridization and geometry around the indicated carbon (cover bottom) Sapling Hw 1.24. Identify the ...

Label each carbon atom with the appropriate geometry
Solved Label each carbon atom with the appropriate geometry. - Chegg The top carbon has three things bonded to it, so its general formula is AB3, or TRIGON… View the full answer Transcribed image text : Label each carbon atom with the appropriate geometry. What is the geometry around each of the three central atoms in the CH ... Carbon 2. This atom has three atoms directly attached and no lone pairs. Its electron geometry and its molecular geometry are both trigonal planar. Oxygen 3. This atom has two atoms directly attached and two lone pairs. Its electron geometry is tetrahedral but its molecular geometry is bent as in water. (From Meritnation) label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...
Label each carbon atom with the appropriate geometry. Chapter 9 Homework Flashcards - Questions and Answers | Quizlet Recall that bonds require p atomic orbitals, so the maximum hybridization of a C atom involved in a double bond is sp2 and in a triple bond is sp. There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond. Solved Label each carbon atom with the appropriate geometry. | Chegg.com This problem has been solved! Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH linear trigonal pyramidal CH2 tetrahedral trigonal planar ... Assignment Part A - Carbon dioxide transport Drag each label to the ... 4. Carbon dioxide diffuses into alveoli. Carbon dioxide is transported from veins into alveoli along a concentration gradient (high to low concentration). 5. Air exits through mouth and nose. Conclusion. Above is the solution for "Assignment Part A - Carbon dioxide transport Drag each label to the appropriate location on the…". Solved Label each carbon atom with the appropriate geometry. - Chegg Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH linear trigonal planar bent CH2 trigonal pyramidal tetrahedral CH2 С С H ; Question: Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH linear trigonal planar bent CH2 trigonal pyramidal tetrahedral CH2 С С H
(Get Answer) - Label each carbon atom with the appropriate geometry ... Label each carbon atom with the appropriate geometry. Trigonal pyrimidal Trigonal planar Tetrahedral Linear Bent CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (single bond) C (triple bond) CH Finding the hybridization of atoms in organic molecules ... - Khan Academy Worked examples: Finding the hybridization of atoms in organic molecules. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedral ČH2 trigonal pyramidal linear bent CH ↑ ↑ ↑ ↑. Mastering Chemistry Tro Chapter 10 Flashcards - Quizlet According to the electronegativity difference between the atoms in methane, it would be appropriate to label hydrogen with the symbol ____, and carbon with the symbol ____. hydrogen δ0 ; carbon δ0 Examine the three dimensional structures of each of the following molecules in the simulation, which can be found in the Real Molecules mode.
⚗️Label each carbon atom with the appropriate geometry. Bin 1 points to ... Label each carbon atom with the appropriate geometry. Bin 1 points to a carbon bonded to a double bonded carbon and single bonded to two hydrogens. Bin 2 points to a carbon double bonded to a carbon and single bonded to a carbon and one hydrogen. Bin 3 is a carbon single bonded to two carbons and single bonded to two hydrogens. Carbon dioxide (CO2) lewis dot structure, molecular geometry, bond ... The electron geometry of CO2 is also linear. In the CO2 lewis structure, there is a total of 4 lone pairs present. Two lone pairs on each oxygen atom. The bond angle of CO2 is 180º. Since it is linear in shape with an arrangement like that O=C=O. Two types of hybridization in CO2 - Sp, and Sp2. Name the geometry around each carbon atom. What is the ... - OneClass Below is the Lewis structure of cyclohexane (C 6 H 12) molecule, a cyclic compound used in the manufacture of nylon and found in the distillation of petroleum.. Name the geometry around each carbon atom. What is the hybridization of each carbon atom? Solved Label each carbon atom with the appropriate geometry. - Chegg This problem has been solved! Label each carbon atom with the appropriate geometry. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Label each carbon atom with the appropriate geometry.
Answered: Predict the approximate molecular… | bartleby Solution for Predict the approximate molecular geometry around each carbon atom of acetonitrile: H3C C N: right C atom left C atom linear linear bent bent…
Label each carbon atom with the appropriate geometry. - Transtutors Label each carbon atom with the appropriate geometry. Trigonal pyrimidal Trigonal planar Tetrahedral Linear Bent CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (single bond) C (triple bond) CH
Solved Label each carbon atom with the appropriate | Chegg.com Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Question : Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 This problem has been solved!
Label each carbon atom with the appropriate geometry. - OneClass 5 Nov 2019. Label each carbon atom with the appropriate geometry. Trigonal pyrimidal. Trigonal planar. Tetrahedral. Linear. Bent. CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (singlebond) C (triple bond) CH. Show full question.
Answered: Label each carbon atom with the… | bartleby Solution for Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH tetrahedral linear trigonal planar bent CH2 trigonal pyramidal CH2 C C H.
label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...
What is the geometry around each of the three central atoms in the CH ... Carbon 2. This atom has three atoms directly attached and no lone pairs. Its electron geometry and its molecular geometry are both trigonal planar. Oxygen 3. This atom has two atoms directly attached and two lone pairs. Its electron geometry is tetrahedral but its molecular geometry is bent as in water. (From Meritnation)
Solved Label each carbon atom with the appropriate geometry. - Chegg The top carbon has three things bonded to it, so its general formula is AB3, or TRIGON… View the full answer Transcribed image text : Label each carbon atom with the appropriate geometry.

















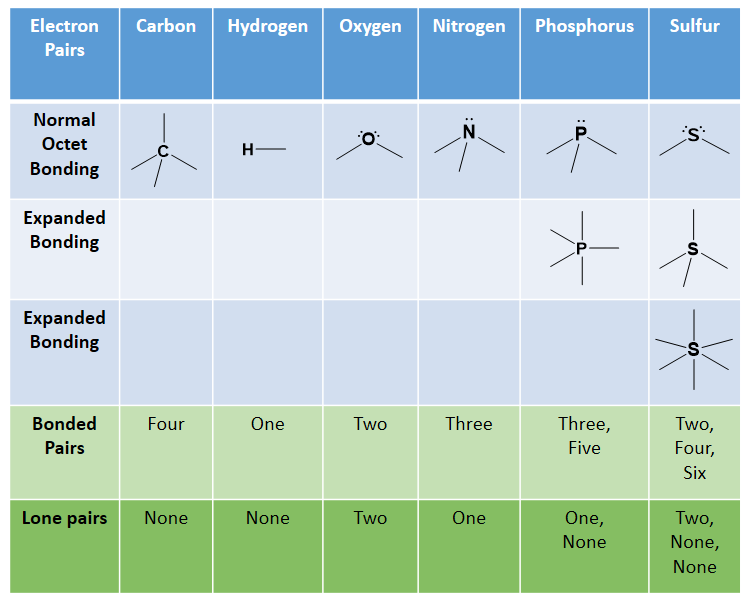
Post a Comment for "41 label each carbon atom with the appropriate geometry"